| Electrolytic Cells | 您所在的位置:网站首页 › positive charged › Electrolytic Cells |
Electrolytic Cells
|
Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they are not the only kind of electrochemical cell. The reverse reaction in each case is non-spontaneous and requires electrical energy to occur. IntroductionThe general form of the reaction can be written as: \[ \underset{\longleftarrow \text{Non spontaneous}}{\overset{\text{Spontaneous} \longrightarrow}{\text{Reactants} \rightleftharpoons \text{Products} + \text{Electrical Energy}}} \nonumber \] It is possible to construct a cell that does work on a chemical system by driving an electric current through the system. These cells are called electrolytic cells. Electrolytic cells, like galvanic cells, are composed of two half-cells--one is a reduction half-cell, the other is an oxidation half-cell. The direction of electron flow in electrolytic cells, however, may be reversed from the direction of spontaneous electron flow in galvanic cells, but the definition of both cathode and anode remain the same, where reduction takes place at the cathode and oxidation occurs at the anode. Because the directions of both half-reactions have been reversed, the sign, but not the magnitude, of the cell potential has been reversed. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. The main differences are outlined below:
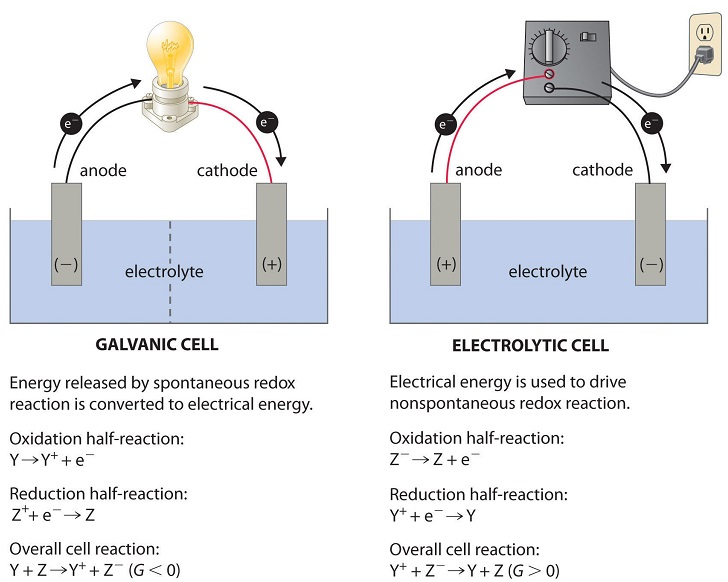
Figure \(\PageIndex{1}\): Electrochemical Cells. A galvanic cell (left) transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work. The oxidative and reductive half-reactions usually occur in separate compartments that are connected by an external electrical circuit; in addition, a second connection that allows ions to flow between the compartments (shown here as a vertical dashed line to represent a porous barrier) is necessary to maintain electrical neutrality. The potential difference between the electrodes (voltage) causes electrons to flow from the reductant to the oxidant through the external circuit, generating an electric current. In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; only a single compartment is employed in most applications. In both kinds of electrochemical cells, the anode is the electrode at which the oxidation half-reaction occurs, and the cathode is the electrode at which the reduction half-reaction occurs. Table \(\PageIndex{1}\): Properties of Galvanic and Electrochemical Cells Electrochemical cell (Galvanic Cell) Electrolytic cell A Galvanic cell converts chemical energy into electrical energy. An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. The two half-cells are set up in different containers, being connected through the salt bridge or porous partition. Both the electrodes are placed in a same container in the solution of molten electrolyte. Here the anode is negative and cathode is the positive electrode. The reaction at the anode is oxidation and that at the cathode is reduction. Here, the anode is positive and cathode is the negative electrode. The reaction at the anode is oxidation and that at the cathode is reduction. The electrons are supplied by the species getting oxidized. They move from anode to the cathode in the external circuit. The external battery supplies the electrons. They enter through the cathode and come out through the anode. Electrolytic CellsTo explain what happens in an electrolytic cell let us examine the decomposition of molten sodium chloride into sodium metal and chlorine gas. The reaction is written below. ---------> Non spontaneous ( electrolytic cell ) 2 Na Cl (l) \(\rightleftharpoons\) 2 Na (s) + Cl2 (g) |
【本文地址】